Miguel Gago, Raquel Costa, Miguel Fonseca, Joana Almeida, Helena Brigas, Ghazal Banisadr, José-Francisco Rocha, Joerg Holenz, Joaquim Ferreira
Joaquim J. Ferreira, Olivier Rascol, Fabrizio Stocchi, Angelo Antonini, Paloma Lapuente, Guillermo Castilla-Fernández, Helena Brigas, Ghazal Banisadr, José-Francisco Rocha, Joerg Holenz, Werner Poewe
Joaquim J. Ferreira1, Jee-Young Lee, Hyeo-il Ma, Beomseok Jeon, Werner Poewe, Angelo Antonini, Fabrizio Stocchi, Daniela M. Rodrigues, Stanley Fisher, Miguel M. Fonseca, José-Francisco Rocha, Guillermo Castilla-Fernández, Joerg Holenz, Olivier Rascol
Daniel Kremens, Fahd Amjad, Sandeep Thakkar, Bita Naderi, Diogo Martins, José-Franciso Rocha, Ghazal Banisadr, Stanley Fisher

Jee-Young Lee, Joaquim J. Ferreira, Hyeo-il Ma, José-Francisco Rocha, Beomseok Jeon, on behalf of Korean OGT_001 study investigators

Joaquim Ferreira, Andrew Lees, Ana Santos, Roberto Pinto, Nelson Lopes, Teresa Nunes, Jose Rocha and Patrício Soares-da-Silva
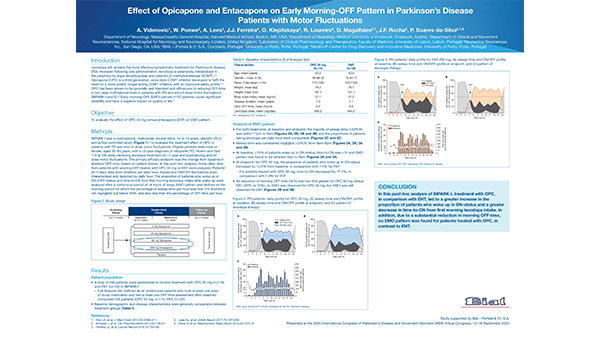
A. Videnovic, W. Poewe, A. Lees, J.J. Ferreira, O. Klepitskaya, R. Loureiro, D. Magalhaes, J.F. Rocha, P. Soares-da-Silva
