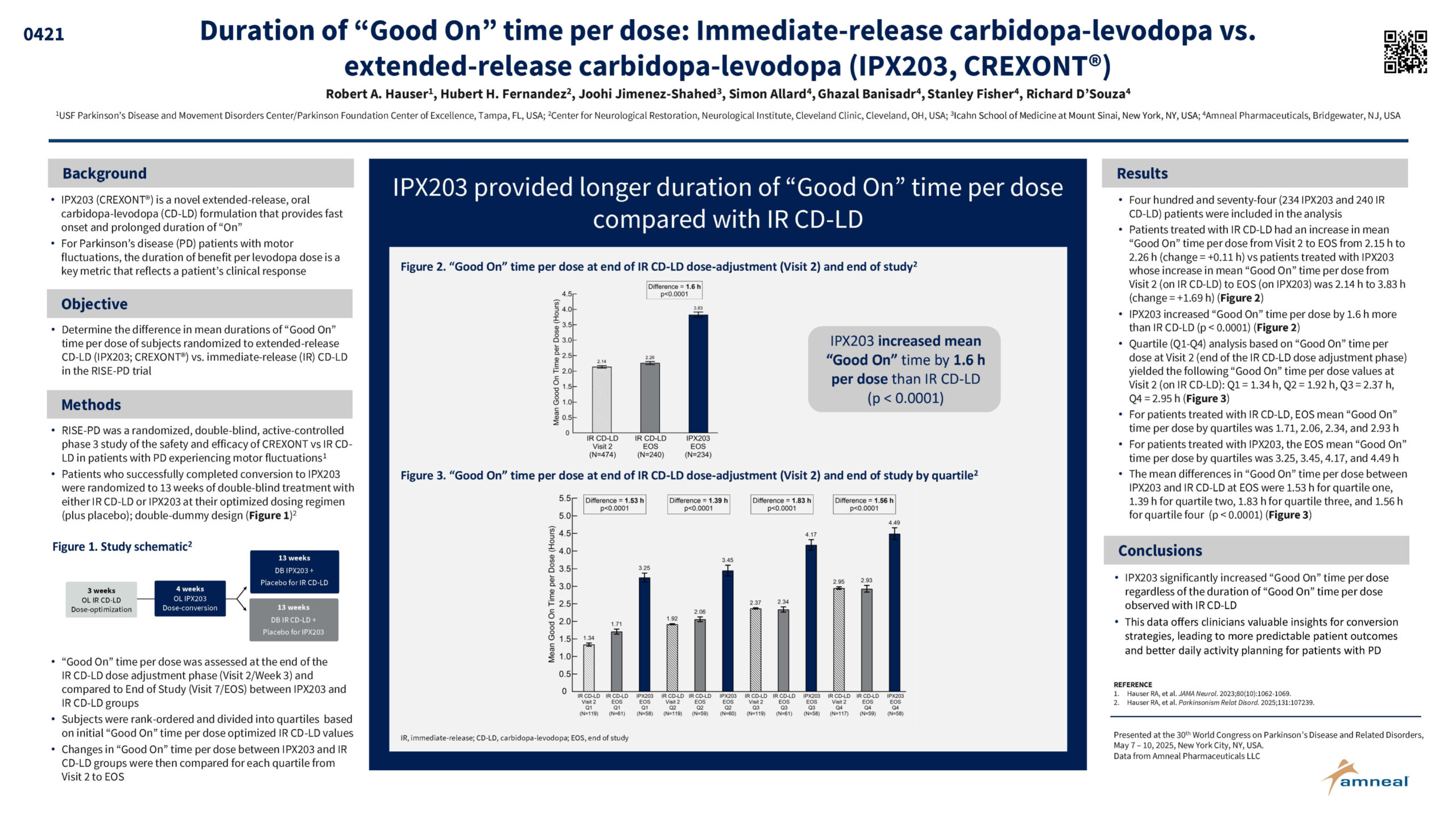
Robert A. Hauser, Hubert H. Fernandez, Joohi Jimenez-Shahed, Simon Allard, Ghazal Banisadr, Stanley Fisher, Richard D’Souza
Stuart H. Isaacson, Hubert H. Fernandez, Camilla Kilbane, Ghazal Banisadr, Arkadiy Pitman, Stanley Fisher, Richard D’Souza
Robert A. Hauser, Simon Allard, Ghazal Banisadr, Stanley Fisher

Robert A. Hauser, Ghazal Banisadr, Stanley Fisher, Hester Visser, Richard D’Souza

Robert A. Hauser, Alberto J. Espay, William Ondo, Beth Safirstein, Henry Moore, Rajeev Kumar, Ghazal Banisadr, Stanley Fisher
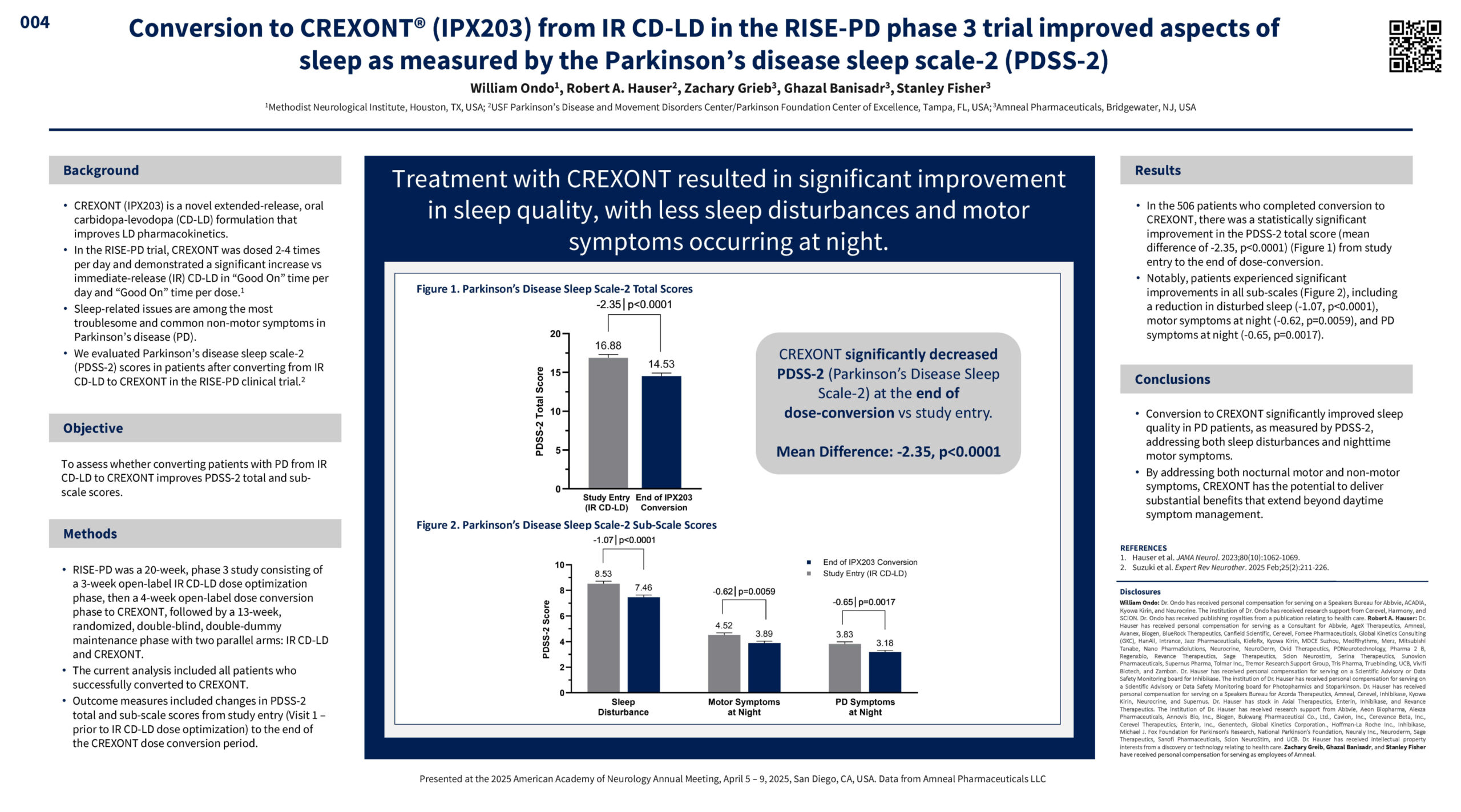
William Ondo, Robert A. Hauser, Zachary Grieb, Ghazal Banisadr, Stanley Fisher
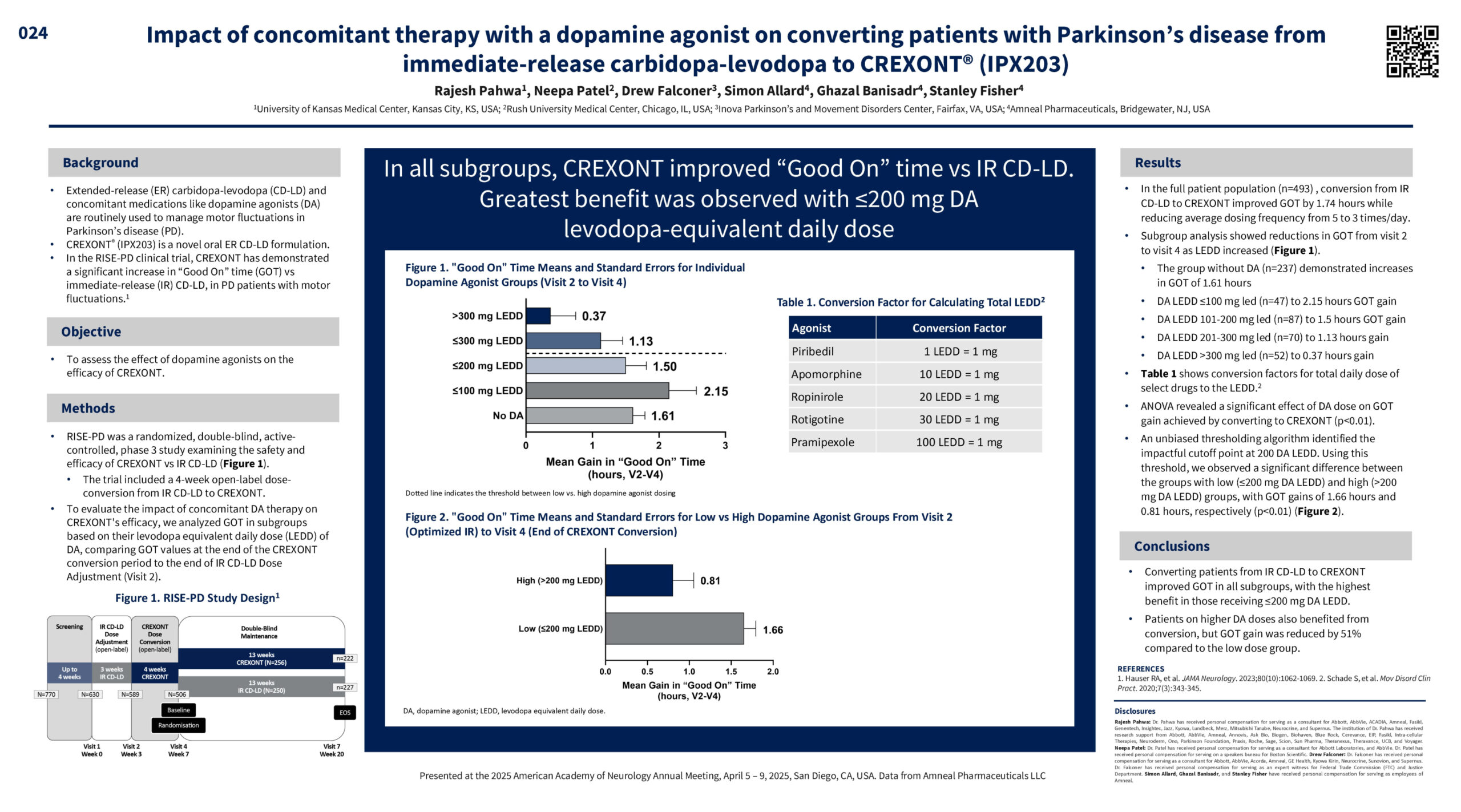
Rajesh Pahwa, Neepa Patel, Drew Falconer, Simon Allard, Ghazal Banisadr, Stanley Fisher
Hubert H. Fernandez, Robert A. Hauser, Vanessa K. Hinson, Nirav Pavasia, Eric Molho, Leonid Zeitlin, Hester Visser, Richard D’Souza

Robert A. Hauser, Hubert H. Fernandez, Kevin Klos, Susan Criswell, Neepa Patel, Ghazal Banisadr, Stanley Fisher
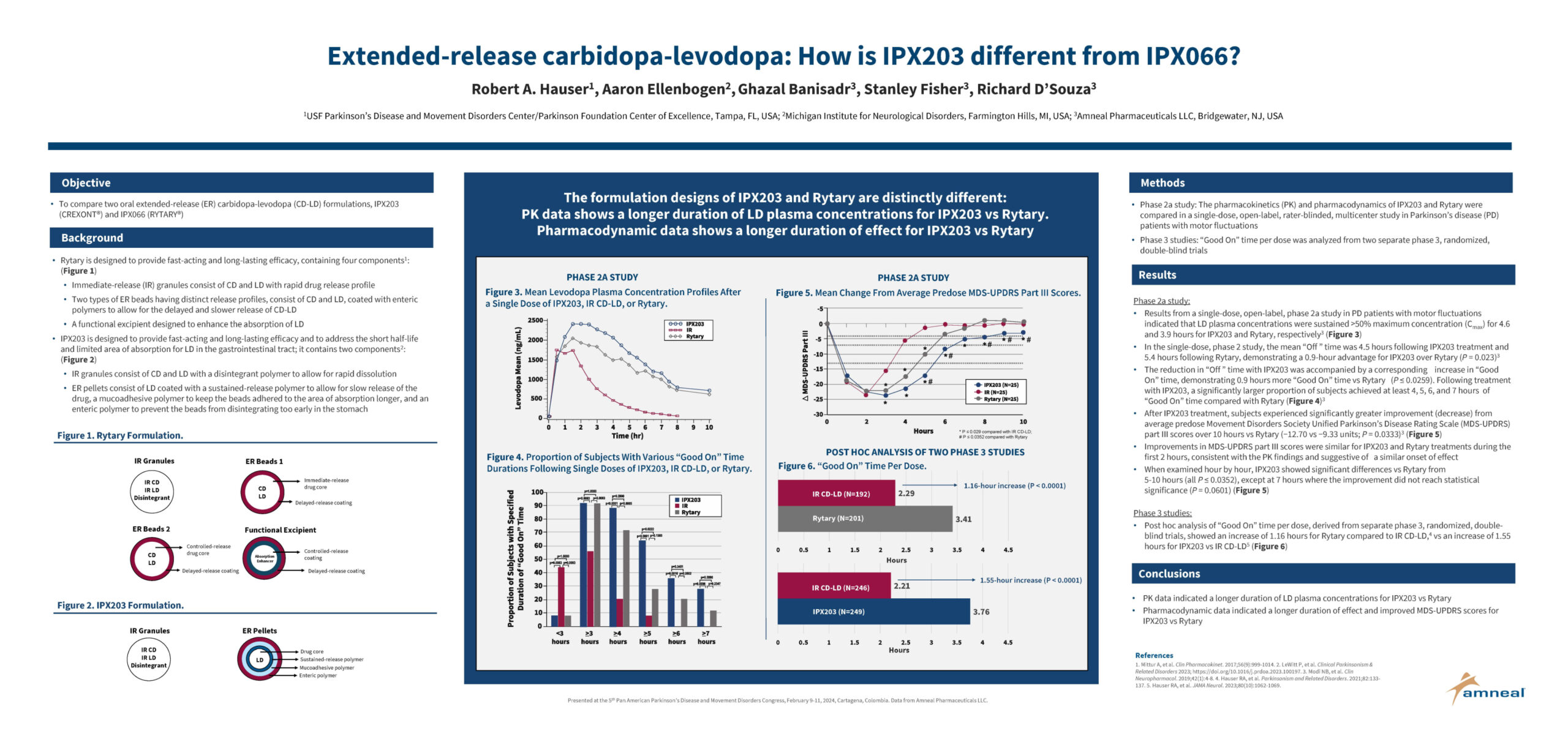
Robert A. Hauser, Aaron Ellenbogen, Ghazal Banisadr, Stanley Fisher, Richard D’Souza
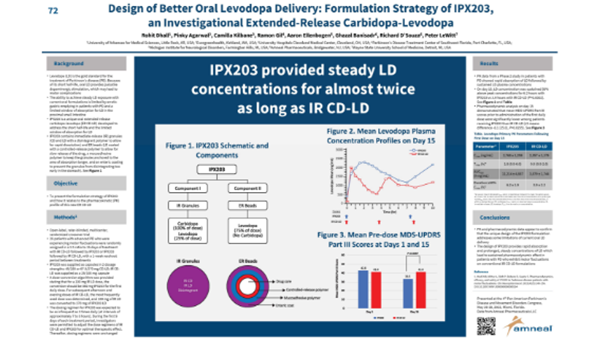
Rohit Dhall, Pinky Agarwal, Camilla Kilbane, Ramon Gil, Aaron Ellenbogen, Ghazal Banisadr, Richard D’Souza, Peter LeWitt